Aseptic Transfer: the fragile points of sterility Where process design determines whether sterility holds - or fails

In biotech and biologics manufacturing, aseptic transfer is rarely compromised by a single failure.
It becomes fragile at specific points of the process, where physical limits, microbiological risks and system design converge. These vulnerabilities do not appear at the end of the journey: they are built into the process itself, step by step.
This article does not aim to document every technical detail of aseptic transfer. Instead, it focuses on why certain phases represent points of fragility and how process decisions determine whether sterility can be consistently maintained.
By reading the process through its most exposed moments, we explore how regulatory requirements translate into concrete process responsibilities, and why robust sterility depends less on reaching target conditions than on governing physical transitions.
Aseptic Transfer is far from a simple handling procedure. It is an integrated ecosystem where the drug product and its primary components, the same syringes, needles or tubing that will later come into direct product contact, travel along parallel paths that must converge in Grade A without ever compromising system integrity. Under the rigorous mandates of Annex 1, this convergence has become a challenge of millimetric precision: from managing endotoxins during the initial wash to mastering steam physics within sterilization loads.
.png?width=750&height=550&name=Immagine-Articolo_ABM2.0_AsepticTrasnfer_Fedegari_2026_v3.0%20(1).png)
The anatomy of Aseptic Transfer
A robust Aseptic Transfer is not a checklist, it is an integrated ecosystem.
Components enter the facility contaminated from the external manufacturer and must undergo a rigorous process:
Validated washing: the first step in the prevention of microbiological, particulate and pyrogenic contamination
Packaging strategy: products are prepared for sterilization using Tyvek® bags or stainless steel canisters, depending on a risk assessment process
Steam sterilization: the core phase where sterilization happens inside the autoclave using saturated steam
Aseptic transfer: the movement of sterile parts from the autoclave to the Grade A isolator via a Rapid Transfer Port (RTP)
After all these steps, the sterile process is completed. But for the chain to hold, every single link must be unbreakable, starting from the very first one: washing.
Washing: the critical barrier against contamination
Washing is often underestimated in aseptic transfer strategies.
The revised EU GMP Annex 1 (2022) has made this explicit: cleaning is a Critical Control Point within the Aseptic Transfer cycle.
Why is this phase so vital? This is where aseptic transfer becomes fragile. Because sterilization cannot compensate for poor cleaning. Endotoxins, for example, are heat-stable and are not reliably inactivated by standard saturated steam cycles. Therefore, washing is the only effective means of endotoxin control for product-contact parts.
An effective, validated washing cycle:
- • removes particles, toxins and process residues
- • drastically lowers the bio-burden prior to sterilization
- • improves the robustness of the downstream sterilization process
Deep dive into washing’s crucial role
Carlo Cattenati, Product Manager at Fedegari and Alessia Cina, Training and process development expert at Fedegari Americas talked extensively about this topic in our latest live webinar, “Washing the hidden key to sterility”, that we also analyzed in our blog.
Check out our resources:
[watch the webinar recording] or read our [latest blog post]
Annex 1 towards closed systems
In the context of pharmaceutical production, Annex 1 stands as the primary regulatory reference. Among its directives regarding product sterilization, it strongly encourages the use of closed systems to protect both the sterile product and the environment.
Closed systems guarantee:
- • the same sterile barrier as product-contact parts
- • a reduced need for additional sanitization
- • lower cumulative contamination risk
This regulatory stress on closed systems directly affects the choice of sterilization techniques and aseptic transfer methods, pushing the industry to evaluate packaging solutions more critically.
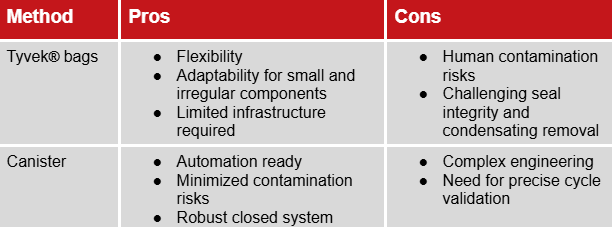
Tyvek® bags of Canisters? Choosing the right packaging
Once washed, components must be packaged to maintain sterility until they reach the filling station. The choice between Tyvek® bags and Canisters is a strategic, risk-based decision that impacts compliance and the final outcome of the sterility assurance level.
Tyvek® bags offer simplicity and flexibility, especially for small, irregular components and require limited infrastructure. However, the manual bag removal introduces a "human variable" which increases the risk of contamination. Furthermore, ensuring seal integrity and condensate removal can be challenging.

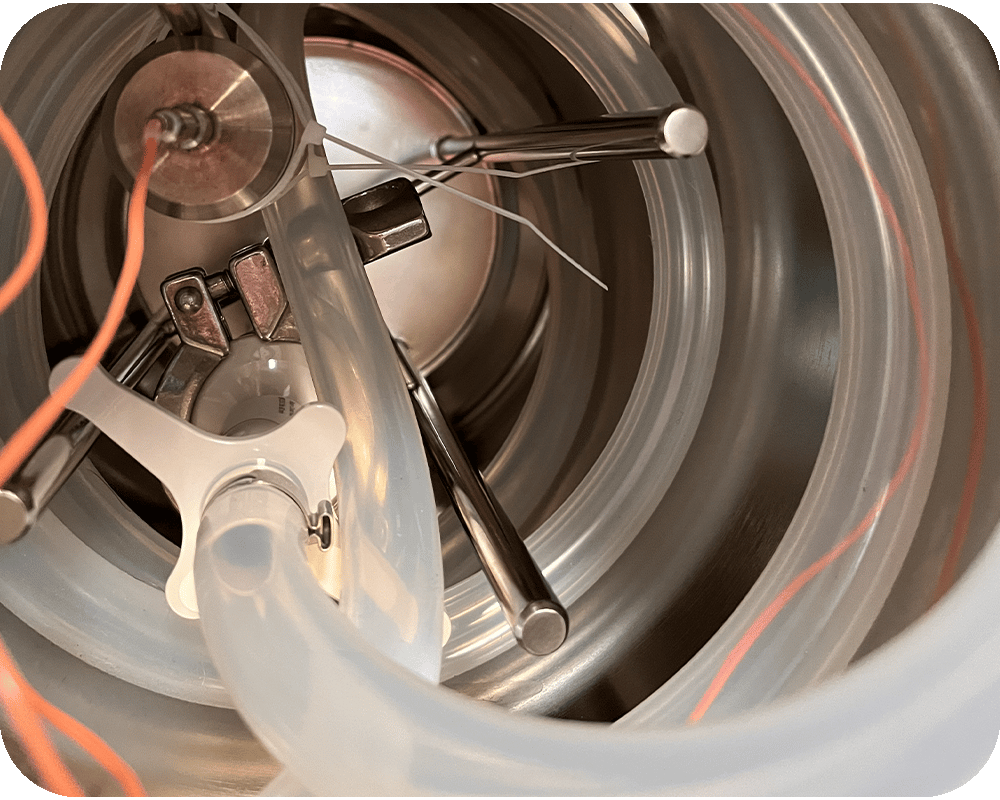
Canisters align perfectly with the "closed system" philosophy posed by Annex 1 and minimize human intervention, thus limiting contamination risks. However, they present an increased engineering complexity and this is where steam sterilization becomes deceptively complex: because of their mass, geometry and condensate behavior, they are difficult to penetrate with heat. They require a sterilization cycle that is not just "run”, but scientifically designed.
The comparison at a glance:

Canisters: where sterilization challenges become visible
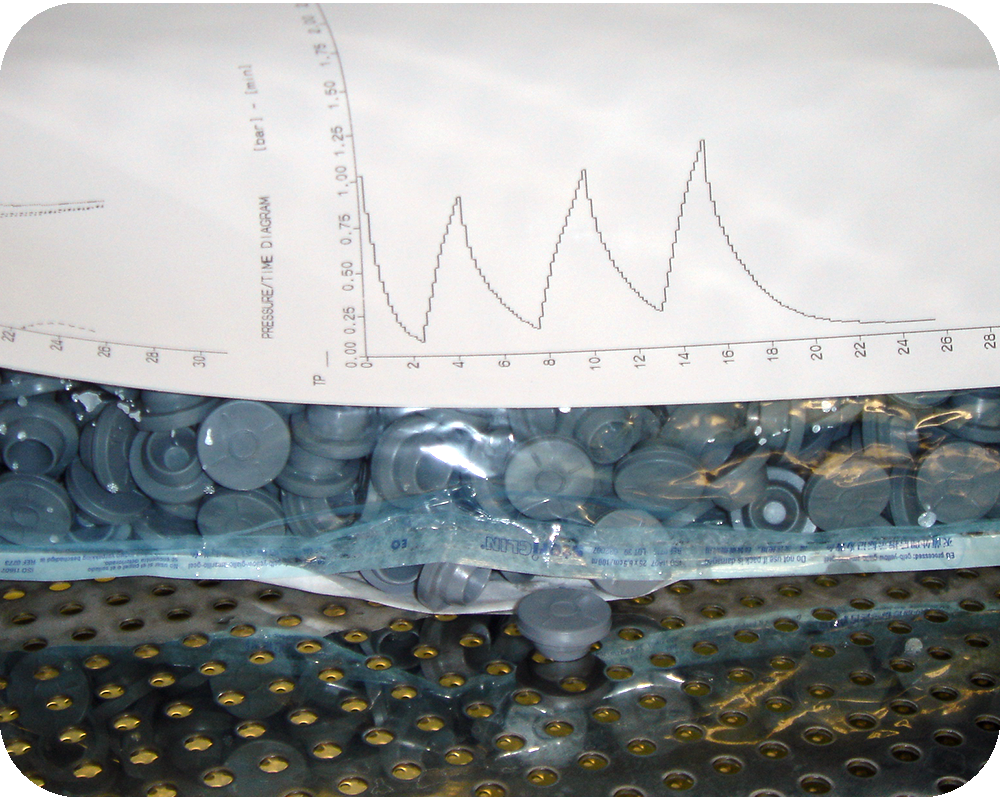
The canister does not create new sterilization problems, it makes existing ones impossible to ignore.
In rigid, closed systems, phenomena such as air removal, condensate formation and temperature uniformity are fully exposed. These challenges exist in many sterilization scenarios, but in a canister they cannot be absorbed or compensated by the process.
This is because, in a canister, the inlet for steam and the outlet for air are the same element: the filter. Air removal, steam admission and pressure release all occur through a single interface, making system behavior extremely sensitive to how physical transitions are managed.
In this context, sterilization is no longer about reaching a target temperature. It is about governing how the system moves through physical transitions: from air to steam, from liquid to vapor, and from heating to drying.
System integrity becomes part of this equation. Vacuum application is not neutral: overly aggressive vacuum can damage filters and compromise the sterile boundary, while slow, modulated vacuum becomes a deliberate process choice to preserve integrity.
For this reason, the canister makes one thing very clear: robust sterilization depends on governing physical transitions throughout the cycle, not simply on exposure to sterilization conditions.
A holistic path to sterility
Washing, packaging, sterilization and transfer form a single, interdependent process. When these interactions are not governed, fragility emerges — even in processes that appear compliant.
Whether components are packaged in Tyvek® bags or transferred through canisters, the objective remains the same: preserving sterility by governing air, condensate, pressure and physical transitions across the entire process.
Understanding where sterility becomes fragile is a prerequisite for process robustness over time.
Visualize the Aseptic Transfer
To support this process-level understanding, we created an infographic that provides a clear, high-level view of the aseptic transfer flow, highlighting:
- • The six critical stations of the process
- • Where sterility is most exposed
- • How Tyvek® bags and Canisters compare from a risk perspective
A practical tool to align technical, quality and engineering teams around the same process view.